akbeemer
SURVIVOR
Huh ?? ? WTF Over?
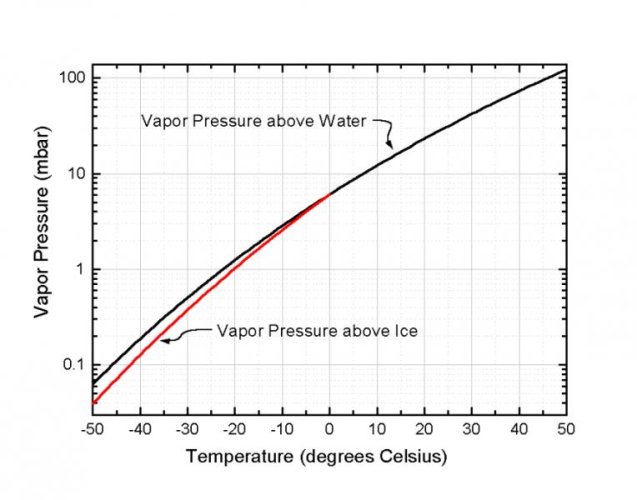
I don't understand your point Paul. Yes, I identified the formula PV=nRT incorrectly. It is the ideal gas law not Boyle's law. However, H2O in a tire can change from a liquid state to a vapor and back depending on temperature and pressure. This change in state can result in pressure fluctuations that are larger than with "dry" nitrogen.